Wainua (eplontersen) for FAP
What is Wainua for FAP?
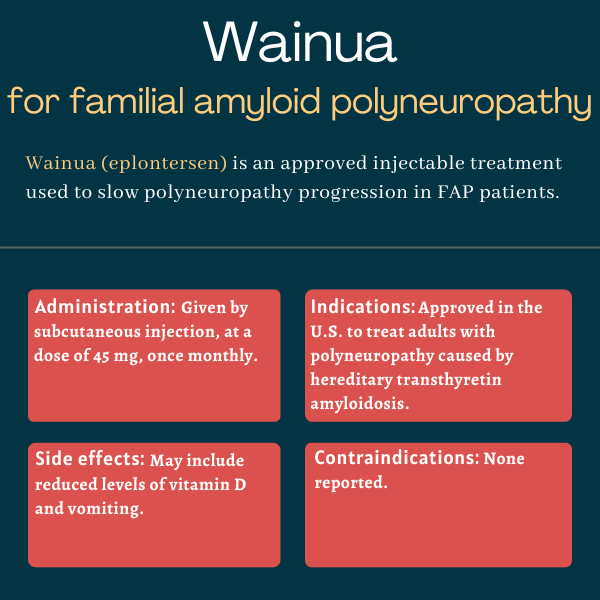
Wainua (eplontersen) is an under-the-skin, or subcutaneous, injectable therapy approved in the U.S. for adults with familial amyloid polyneuropathy (FAP), also known as hereditary transthyretin amyloidosis with polyneuropathy.
Designed to prevent nerve damage and thereby slow or halt disease progression, Wainua — which is sold as Wainzua in Europe — became the first approved FAP therapy that can be self-administered by patients through an autoinjector.
Formerly known as IONIS-TTR-LRx and AKCEA-TTR-LRx, Wainua was discovered by Ionis Pharmaceuticals and later developed in collaboration with AstraZeneca. The therapy is marketed by both companies in the U.S., while AstraZeneca holds exclusive commercialization rights in all countries outside the U.S.
Wainua also is being developed as a potential treatment for transthyretin amyloid cardiomyopathy, an FAP-related condition that’s characterized mainly by heart damage.
Therapy snapshot
| Brand name: | Wainua |
| Chemical name: | Eplontersen |
| Usage: | Used for the treatment of adults with FAP; can slow or halt disease progression |
| Administration: | Subcutaneous injection |
How does Wainua work?
FAP is caused by mutations in the TTR gene, which provides instructions for making transthyretin (TTR), a protein involved in the transport of vitamin A and the thyroxine hormone throughout the body.
These mutations result in the production of an abnormal form of TTR that’s prone to form toxic clumps called amyloids. Such amyloids build up in tissues, mostly in the peripheral nerves — those outside the brain and spinal cord — but also in the heart. This leads to polyneuropathy, or damage of many peripheral nerves, and in some cases, cardiomyopathy, or damage to the heart, causing FAP symptoms.
Wainua is an RNA-targeted therapy that works by binding to and blocking TTR’s messenger RNA, an intermediate molecule derived from DNA that serves as a template for protein production. As such, the therapy is expected to reduce the production of all forms of the TTR protein and prevent further buildup of toxic TTR clumps, ultimately slowing FAP progression.
The therapy’s mechanism of action is similar to that of Tegsedi (inotersen), an older FAP therapy also developed by Ionis. However, the second-generation treatment was modified to boost its entry into liver cells, the body’s main transthyretin producers. This allows Tegsedi’s successor to be administered less frequently — once a month versus once a week with Tegsedi — likely reducing the treatment burden for patients.
Who can take Wainua?
Wainua was approved by the U.S. Food and Drug Administration (FDA) in December 2023 for the treatment of adults with polyneuropathy caused by hereditary transthyretin amyloidosis. The decision made Wainua the first and only therapy that can be self-administered by patients via an autoinjector.
A support program, called Wainua Way, is available to help patients in the U.S. learn how to administer Wainua and make the medication more affordable. AstraZeneca Access 360, a part of this program, can help answer patient questions regarding insurance coverage, out-of-pocket costs, and financial assistance programs.
The therapy is also cleared in Canada and the U.K. for the same indication as in the U.S., while its approval in the Euopean Union covers adults with stage 1 or 2 FAP.
Who should not take Wainua?
Wainua’s prescribing information does not list any contraindications, or situations in which the therapy should not be administered to patients.
How is Wainua administered?
Wainua is available as a single-dose autoinjector containing 0.8 mL of a clear, colorless-to-yellow solution with the recommended dose of 45 mg eplontersen. Eplontersen is the therapy’s active ingredient.
The medication is given via a single subcutaneous injection on the same day of each month. It can be administered at home or on the go by patients themselves and/or by caregivers after appropriate training by a healthcare provider. Additional instructions for using Wainua can be found on the treatment’s website.
Wainua is injected into the abdominal area (stomach) or front of the thighs. The back of the upper arm also can be used as an injection site if the medication is administered by a caregiver or a healthcare professional.
Prior to administration, the autoinjector should be removed from the refrigerator and allowed to warm at room temperature for about 30 minutes; no other warming methods should be used. If needed, Wainua can be stored at room temperature for up to six weeks.
The injection site — which should not have scars or be bruised, red, tender, or hard — should first be disinfected. The autoinjector should be held against the skin for 10 seconds after the start of the injection.
If a planned dose of Wainua is missed, a new dose should be given as quickly as possible, and monthly dosing should resume from that new date.
Wainua in clinical trials
Wainua’s approval in the U.S. was based mainly on interim 8-month data from a global, Phase 3 trial called NEURO-TTRansform (NCT04136184).
NEURO-TTRansform
The Ionis-sponsored NEURO-TTRansform study enrolled 168 adults with stage 1 or 2 FAP and with mild to moderate nerve damage symptoms. A total of 144 participants were originally assigned to receive the therapy, once a month, and most completed about 15 months of treatment.
The trial’s main goals were to assess changes in blood TTR levels and in two separate disease measures. One, the Neuropathy Impairment Score +7 (mNIS+7), evaluates nerve damage-related disability, while the other, called the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QoL-DN), measures the impact of nerve damage on quality of life. In both mNIS+7 and Norfolk QoL-DN, higher scores indicate worse disease severity and disability.
The results were compared with those from 60 FAP patients given a placebo in a previous Phase 2/3 trial (NCT01737398) that helped support Tegsedi’s approval.
A pre-planned interim analysis after 35 weeks, or about eight months, of treatment showed that Wainua significantly reduced blood TTR levels by 81.2% compared with a 14.8% drop in the external placebo group.
The therapy also was significantly superior to a placebo at slowing disease progression, with Wainua-treated patients experiencing a significantly lower increase in mNIS+7 scores (average of 0.2 vs. 9.2 points).
About eight months of Wainua treatment also was associated with a 3.1-point reduction in Norfolk QoL-DN scores, indicating better quality of life; those given a placebo showed a score increase of 8.7 points.
Subsequent data over 15 months of treatment confirmed Wainua’s superior benefits over the external placebo. Use of the therapy resulted in a significantly greater reduction in TTR levels (81.7% vs. 11.2%) and in significantly smaller increases in mNIS+7 scores (0.3 vs. 25.1 points). Treatment with Wainua also continued to result in a reduction in Norfolk QoL-DN scores (by 5.5 points), indicating better life quality, compared with an increase of 14.2 points in the placebo group.
In addition, nearly half (47%) of Wainua-treated patients experienced less nerve damage-related disability, and slightly more than half (58%) reported quality of life improvements after 15 months of treatment. This was in comparison with 17% and 20% of placebo patients, respectively.
An exploratory analysis at 15 months also suggested that Wainua may stabilize or improve heart function in FAP patients with cardiomyopathy.
Moreover, NEURO-TTRansform’s longer-term data suggested the therapy’s effects were sustained for more than 1.5 years of treatment.
Ongoing studies
Participants completing the NEURO-TTRansform trial could enroll in an ongoing open-label extension study (NCT05071300), in which they receive Wainua for up to three more years. That study, which also includes patients who participated in prior Tegsedi trials, aims to evaluate Wainua’s long-term safety and efficacy. It’s expected to end in 2024.
Common side effects of Wainua
The most commonly reported side effects with Wainua in clinical trials include:
- reduced blood vitamin A levels
- vomiting.
Vitamin A supplementation
Because Wainua can cause a decrease in vitamin A levels in the blood, including drops below the normal range, it’s recommended that patients on the therapy take supplements of the recommended daily allowance of vitamin A. Importantly, doses higher than recommended should not be given even if vitamin A levels in blood remain low, as these do not reflect the vitamin’s total levels in the body.
If patients on Wainua develop eye-related issues suggestive of vitamin A deficiency, such as dry eyes or problems seeing at night or in dim light, they should inform their physician and be referred to an ophthalmologist.
Use in pregnancy and breastfeeding
There are no data on the use of Wainua in people who are pregnant or breastfeeding. Vitamin A is key for normal fetal development, and changes in its levels may cause developmental defects. Still, in mouse studies, the therapy did not cause notable complications with fetal development when administered during pregnancy.
It also is not known if Wainua can be found in human breast milk and whether it has any impact on nursing infants or on milk production.
Patients who are pregnant or nursing, or plan to become pregnant or breastfeed, should discuss this topic with their healthcare team, carefully weighing the potential risks and benefits of continuing or stopping treatment.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Liver transplant, therapies mean longer life with hATTR, data show
- Onpattro stabilizes FAP progression in 2 common genetic variants
- New study tests nucresiran’s potential to slow nerve damage
- Our role as patient advocates is vital, but funding remains an issue
- Gene therapy study placed on hold after elderly man passes away
Related articles

 Fact-checked by
Fact-checked by