Tegsedi (inotersen) for FAP
Last updated March 12, 2024, by Marisa Wexler, MS

What is Tegsedi for FAP?
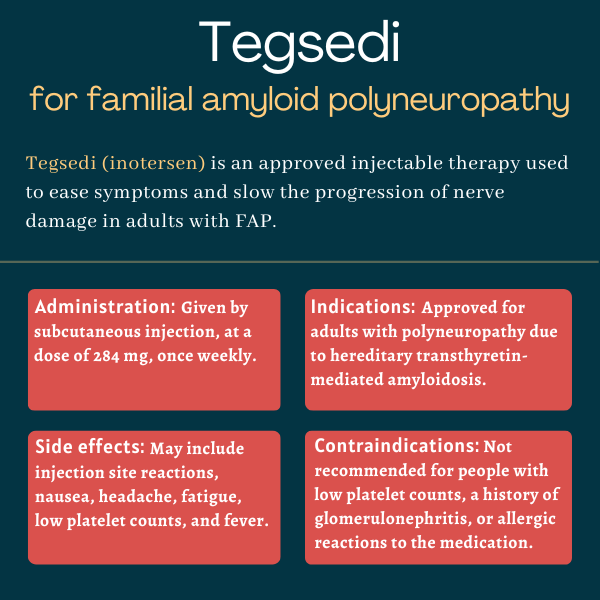
Tegsedi (inotersen) is an approved injection therapy used to ease disease symptoms and slow the progression of polyneuropathy, or damage to multiple nerves, in adults with familial amyloid polyneuropathy (FAP).
Previously known as IONIS-TTRRx, the therapy was developed by Ionis Pharmaceuticals and its now wholly-owned subsidiary Akcea Therapeutics, but is commercialized by Sobi in North America and Europe. It is administered by an injection under the skin, or subcutaneously.
Therapy snapshot
| Brand name: | Tegsedi |
| Chemical name: | Inotersen |
| Usage: | Used to ease symptoms and slow or halt disease progression in adults with FAP |
| Administration: | Subcutaneous injection |
How does Tegsedi work?
FAP, also known as hereditary transthyretin amyloidosis with polyneuropathy, is an inherited progressive condition caused by mutations in the TTR gene, which contains the information needed to produce the transthyretin (TTR) protein.
These mutations result in an abnormal version of TTR being produced and toxic aggregates, called amyloids, accumulating mostly in the peripheral nerves, but also in the heart muscle. The peripheral nerves are located outside the brain and spinal cord and relay sensory and motor information.
Over time, these toxic TTR clumps damage these tissues and lead to FAP symptoms.
For a protein to be produced, the coding gene is first copied into an intermediary molecule called a messenger RNA, or mRNA. This molecule is then read by the cell’s protein-making machinery to create the corresponding protein. Tegsedi is designed to interfere with this process to prevent amyloid deposits from building up.
Belonging to a class of therapies called antisense oligonucleotides (ASOs), Tegsedi is a chemically modified RNA molecule that binds to TTR’s mRNA and promotes its degradation, thereby halting the production of both mutated and normal TTR proteins. By doing so, the therapy is expected to reduce toxic TTR clumps, ease symptoms, and slow or even halt FAP progression.
Who can take Tegsedi?
Tegsedi was approved by the U.S. Food and Drug Administration in October 2018 for treating adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis, another term for FAP. The decision made it the first subcutaneous and self-administered therapy to be approved for FAP.
Due to increased risks of serious bleedings and kidney damage, Tegsedi is only available in the U.S. through a restricted access program called Tegsedi REMS (Risk Evaluation and Mitigation Strategy). This program ensures only certified healthcare professionals and pharmacies can prescribe and sell it, and that patients comply with regular monitoring.
Tegsedi also is approved in Canada and the European Union for adults with FAP who have stage 1 or 2 polyneuropathy.
Who should not take Tegsedi?
Tegsedi is contraindicated, or not recommended, to anyone with:
- fewer than 100 billion platelets — blood clot-promoting components — per liter of blood
- a history of acute glomerulonephritis, a type of kidney inflammation, related to Tegsedi
- a history of an allergic reaction to Tegsedi.
The prescribing label for Tegsedi has a boxed warning for lower than normal platelet counts and glomerulonephritis because these have been reported in patients treated with the medication and may be life-threatening. Patients should be carefully monitored for signs of these conditions.
The therapy also should not be initiated in patients with a urine protein to creatinine ratio of 1,000 mg/g or greater, a sign of kidney damage caused by glomerulonephritis. Tegsedi is also not recommended for patients in whom glucocorticoids and other immunosuppressive treatments are not advised, as these medications may be needed to resolve certain potential side effects of Tegsedi.
How is Tegsedi administered?
Tegsedi is available in single-dose prefilled syringes containing the recommended dose of 284 mg of the active therapy inotersen in 1.5 mL of a clear, colorless-to-pale yellow solution.
The medication is given via a weekly subcutaneous injection, preferably on the same day each week. It can be administered at home by patients or caregivers after appropriate training in how to perform injections. The first injection should be done under medical supervision.
The syringe should be stored in a refrigerator and should be removed and allowed to warm to room temperature for 30 minutes before an injection.
Injections may be given into skin of the abdomen, upper thigh, or upper arm, and injection sites should be rotated between doses. Injections should not be administered into scars, tattoos, or areas of damaged or diseased skin.
If a planned dose is missed, an injection should be given as soon as possible, unless the next scheduled dose is less than two days away. In that case, the missed dose should be skipped and the next dose given as scheduled.
Tegsedi in clinical trials
Approvals of Tegsedi were based primarily on findings from a Phase 3 clinical trial called NEURO-TTR (NCT01737398) and its open-label extension study (NCT02175004).
NEURO-TTR
The international NEURO-TTR investigated the safety and efficacy of Tegsedi in 173 FAP adults, ages 18-82, with stage 1 or 2 polyneuropathy, meaning mild to moderate symptoms. The participants were randomly assigned to receive weekly subcutaneous injections of either Tegsedi or a placebo for about 15 months, or a little over a year.
The study’s main goals were to assess the effects of Tegsedi on nerve damage, or neuropathy, and its impact on quality of life. Neuropathy was measured with the modified Neuropathy Impairment Score +7 (mNIS+7), and quality of life with the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QoL-DN). In both measures, higher scores indicate more advanced disease and greater disability.
Tegsedi significantly reduced the progression of neuropathy and quality of life worsening over 15 months compared with the placebo.
Specifically, Tegsedi-treated patients saw a mean 5.8-point increase in their mNIS+7 scores compared with a 25.5-point gain for those on a placebo — a 19.7-point difference. The therapy also was associated with a 1-point increase in Norfolk QoL-DN versus a 12.7-point-increase with a placebo — a difference of 11.7 points.
Tegsedi’s superiority was observed for most domains of both measures, further supporting its ability to slow polyneuropathy progression in FAP.
The therapy’s beneficial effects were significantly greater than those of a placebo across all patient subgroups, regardless of disease-causing mutations, FAP stage, heart involvement, previous treatment, as well as age, sex, ethnicity, and geographical region.
An additional analysis on other secondary and exploratory efficacy measures also supported Tegsedi for helping to stabilize neuropathy symptoms, especially muscle weakness and loss of sensation.
Extension study
The participants who completed NEURO-TTR had the option to enter an open-label extension study, where all received weekly Tegsedi for up to 260 weeks (about five years) and were monitored for long-term outcomes.
The findings confirmed Tegsedi’s ability to slow polyneuropathy progression and life quality deterioration for more than three years of treatment.
Placebo-treated patients who transitioned to Tegsedi in this study also showed a slowing of disease progression and worsening quality of life, similar to those observed in the active group during NEURO-TTR. Their scores remained generally worse relative to those on Tegsedi from the start, however, indicating that earlier treatment initiation might provide the best long-term outcomes.
Common side effects of Tegsedi
The most common side effects of Tegsedi in clinical trials include:
- injection site reactions
- nausea
- headache
- fatigue
- low platelet counts, or thrombocytopenia
- fever.
Thrombocytopenia
Tegsedi carries a boxed warning noting it may result in lower than normal levels of platelets (thrombocytopenia), which can come on suddenly and cause life-threatening bleeding. As such, the therapy should not be initiated in patients with fewer than 100 billion platelets per liter of blood, and patients on Tegsedi must be routinely monitored to check platelet counts.
If patients notice symptoms of thrombocytopenia, such as unusual bleeding or bruising, they should seek immediate medical care and a platelet count should be obtained as soon as possible. Treatment also should be halted until platelet counts are acceptable levels.
Patients on Tegsedi frequently have platelet counts that can’t be interpreted due to platelets clumping together during testing. If this happens, testing should be repeated using a different anticoagulant to prevent blood clotting in the blood collection tube.
For patients also on antiplatelet medications or anticoagulants, discontinuing these treatments should be considered if platelet counts drop below 50 billion per liter while on Tegsedi.
Glucocorticoid medications are strongly recommended for patients with fewer than 50 billion platelets per liter. Thus, Tegsedi should be avoided for patients for whom such medications are not advised.
Kidney inflammation and damage
Tegsedi also carries a boxed warning for a type of kidney inflammation called glomerulonephritis, which can result in kidney failure and a need for dialysis. Caution is advised when using medications that may affect kidney function.
It’s recommended that patients undergo kidney health tests before starting on Tegsedi and every two weeks while on the therapy. Those with a urine protein to creatinine ratio of at least 1,000 mg/g, indicative of kidney function problems, should not initiate Tegsedi treatment.
If there is suspicion of glomerulonephritis, appropriate testing should be promptly performed to achieve a diagnosis. If confirmed, Tegsedi should be discontinued and immunosuppressive treatment initiated as soon as possible. Patients who cannot take immunosuppressive medications are advised not to initiate Tegsedi.
Stroke
Tegsedi may cause stroke and cervicocephalic arterial dissection, or a tear in a vessel that carries blood to the brain. In clinical studies, one patient had such an event within a couple of days of the first Tegsedi dose. Patients having signs of stroke, including sudden numbness or weakness (especially on one side of the body), confusion, and severe headache or neck pain, should seek immediate medical care.
Inflammatory and immune responses
Like other ASOs, Tegsedi may cause changes in inflammatory and immune activity. This can contribute to immune-related thrombocytopenia and glomerulonephritis, and more rarely to other problems including blood vessel inflammation and neurological issues.
Liver damage
Because ASOs tend to accumulate in the liver, Tegsedi may cause liver injury. Markers of liver damage should be checked upon starting the therapy and then monthly for the duration of treatment.
If patients develop signs of liver dysfunction — including unexplained nausea, vomiting, yellowed skin, and/or dark urine — liver health should be checked at once and Tegsedi should be stopped or discontinued as appropriate.
There have been reports of liver transplant rejection within a few months of starting Tegsedi in people whose transplanted liver had been stable. Markers of liver health should be routinely monitored in these patients and Tegsedi should be discontinued if signs of rejection appear.
Allergic reactions
Tegsedi can cause allergic reactions, which usually occur within a few hours of its administration. Symptoms may include headache, chest pain, chills, flushing, trouble swallowing, involuntary movements, joint or muscle pain, flu-like symptoms, high blood pressure, and reddened palms.
If an allergic reaction occurs, Tegsedi should be discontinued and appropriate supportive care initiated.
Vitamin A supplementation
Due to transthyretin’s essential role in transporting vitamin A throughout the body, Tegsedi may cause blood vitamin A levels to drop below the normal range. It’s recommended that patients on Tegsedi receive daily vitamin A supplements, but the amount taken should never exceed the recommended daily dose (900 micrograms for adult men, 700 micrograms for adult women).
Patients who have eye problems suggestive of vitamin A deficiency, such as vision difficulties at night or in low-light zones, should be referred to an ophthalmologist.
Use in pregnancy and breastfeeding
There is no solid data on the use of Tegsedi by people who are pregnant or breastfeeding, so decisions about its use in these situations need to be made on a case-by-case basis to evaluate the potential risks and benefits.
Vitamin A is needed for fetal development, but the effects of Tegsedi-induced low vitamin A levels on fetal development are unclear. In mouse studies, Tegsedi did not cause any noteworthy problems when administered during pregnancy, but it was associated with adverse fetal outcomes in rabbit studies when given at high doses that were also toxic to the pregnant animals.
An observational study, called TEGSEDI-PRG, is enrolling pregnant women with FAP to assess maternal and fetal outcomes associated with Tegsedi exposure during pregnancy.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- In New Zealand, hope remains despite diagnosis, treatment barriers
- Inflammation markers linked to symptoms in Val30Met-related FAP
- Later FAP symptom onset seen with Ala97Ser mutation in China
- Forging relationships at an amyloidosis conference
- New MRI technique may help detect nerve damage in FAP: Study
Related articles