CRX1008 (tolcapone) for FAP
Last updated March 25, 2024, by Marisa Wexler, MS

What is CRX1008 for FAP?
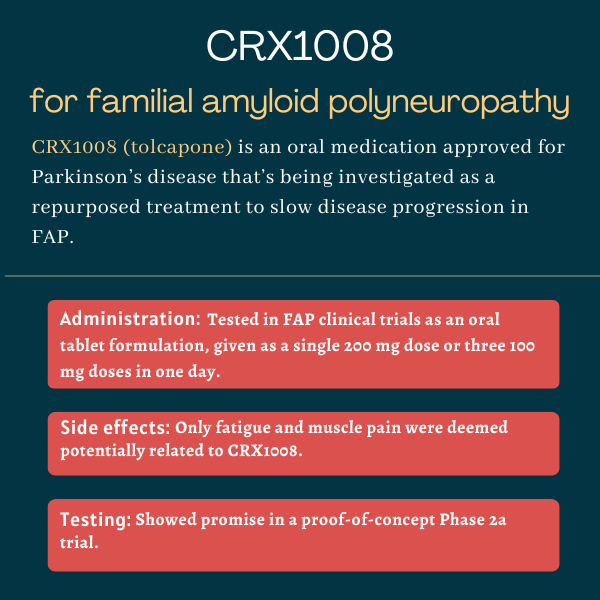
CRX1008 (tolcapone) is an experimental oral therapy that’s being developed to slow or prevent the progression of familial amyloid polyneuropathy (FAP), also known as hereditary transthyretin amyloidosis with polyneuropathy. Polyneuropathy is damage to a person’s peripheral nerves, or those outside the brain and spinal cord.
The treatment’s active ingredient tolcapone is already approved under the brand name Tasmar as an add-on therapy for Parkinson’s disease.
However, therapy showed some promise in stabilizing the transthyretin protein that’s faulty in FAP, leading its original maker SOM Biotech to investigate whether tolcapone could be repurposed to treat FAP and other related conditions. Repositioning existing medicines can shorten a treatment’s development process and lower its cost, because the compound’s safety is already established.
SOM Biotech had named the drug SOM0226, but Corino Therapeutics, which acquired global development and commercialization rights to the therapy in 2017, has since renamed it CRX-1008.
Therapy snapshot
| Treatment name: | CRX1008 (tolcapone) |
| Administration: | Being tested in FAP as an oral tablet formulation |
| Clinical testing: | Completed a proof-of-concept Phase 2a clinical trial |
How does CRX1008 work in FAP?
FAP is caused by mutations in the TTR gene, which provides instructions for making the transthyretin protein, known as TTR. This protein normally helps to carry hormones and vitamin A throughout the body. However, in FAP, patients produce an abnormal version of the protein that ultimately damages several tissues, including the nerves outside the brain and spinal cord.
A healthy TTR protein exists in a tetramer, or a complex of four proteins bound together. The four-protein complex isn’t stable in FAP, so it breaks down into its individual TTR protein components, which are prone to acquire an abnormal structure and form toxic clumps.
Tolcapone, the active agent in CRX1008, has shown the ability to bind and stabilize the TTR tetramer in a way that prevents its dissociation into individual proteins, and is highly effective at stopping the formation of toxic protein clumps. Importantly, this effect was observed with TTR proteins derived from both healthy and mutated TTR genes.
By lowering the amount of TTR protein clumps, the experimental therapy is expected to reduce the nerve damage caused by these clumps, potentially easing symptoms and slowing or halting disease progression in people with FAP.
How will CRX1008 be administered in FAP?
In FAP clinical trials, CRX1008 was tested in the form of film-coated tablets, taken orally either as a single dose of 200 mg or as three doses of 100 mg four hours apart.
CRX1008 in FAP clinical trials
CRX1008’s ability to stabilize the transthyretin protein was assessed in a proof-of-concept Phase 2a clinical trial (NCT02191826) conducted at a single site in Spain.
The study enrolled 11 adults carrying the most common FAP-causing mutation, called Val30Met, and six healthy adults without any TTR mutations. Among those with TTR mutations, three had not yet developed FAP symptoms, three had mild symptoms — known as FAP stage 1 — and the remaining five had moderate symptoms, or were at stage 2.
In the first part of the study, all participants were given a single 200 mg dose of CRX1008, and blood samples were collected at several time points thereafter to assess TTR stabilization. A TTR stabilization value of 20% was considered the minimum expected to provide therapeutic benefit in FAP.
Then, about six weeks later, in the trial’s second part, the participants took three 100 mg tablets of CRX1008 over a day, each separated by four hours. Again regular blood tests were performed after dosing.
The results showed that both the 200 mg and 100 mg doses led to a robust increase in stabilization of blood TTR proteins in all participants. Stabilizing values reached a maximum of 52%, and most participants — 82% in part 1 and 93% in part 2 — reached a TTR stabilization value of 20% or higher.
Pharmacological data indicated that maximum TTR stabilization values were observed two hours after dosing in the trial’s first part. In the second part, maximum values were reached two hours after the first tablet and maintained for up to two hours after the third tablet — in line with treatment blood concentrations being higher during these periods.
These findings demonstrated that maintaining TTR stabilization throughout the day would require either dosing at least three times daily, or designing a delayed-release formulation in which tolcapone could be slowly released into circulation for long periods of time.
Common side effects of CRX1008
In the proof-of-concept Phase 2a trial, the most commonly reported side effects in participants taking CRX1008 were fatigue and muscle pain. However, most were deemed either unrelated or likely unrelated to the therapy. Still, this was a small and short-term study, so more data would be needed to know the possible full effects.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
Related articles