Onpattro (patisiran) for FAP
Last updated Feb. 27, 2024, by Marisa Wexler, MS

What is Onpattro for FAP?
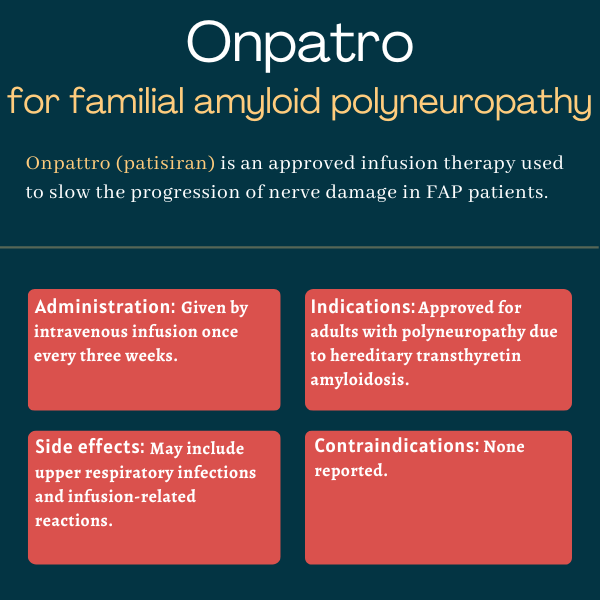
Onpattro (patisiran) is an infusion treatment approved for adults with familial amyloid polyneuropathy (FAP), also known as hereditary transthyretin amyloidosis with polyneuropathy.
The therapy, formerly known as ALN-TTR02, is designed to ease symptoms and slow disease progression. It was developed by Alnylam Pharmaceuticals and is administered to patients via an intravenous (into-the-vein) infusion.
Onpattro also was studied as a potential treatment for transthyretin amyloid cardiomyopathy (ATTR-CM), an FAP-related condition characterized mainly by damage in the heart. However, after the U.S. Food and Drug Administration (FDA) rejected Alnylam’s approval request for ATTR-CM in 2023, the company announced it would no longer pursue this indication for Onpattro in the U.S.
Therapy snapshot
| Brand name: | Onpattro |
| Chemical name: | Patisiran |
| Usage: | Used to treat adults with FAP; can ease symptoms and slow or prevent disease progression |
| Administration: | Intravenous infusion |
How does Onpattro work?
FAP is caused by mutations in the TTR gene, which provides instructions to produce a protein called transthyretin that is involved in the transport of certain molecules, including vitamin A.
These mutations lead to the production of an abnormal transthyretin protein that forms toxic clumps, or aggregates. In FAP, these aggregates mainly accumulate in the peripheral nerves, or those outside the brain and spinal cord, causing polyneuropathy, or damage of many peripheral nerves, and several of the disease’s hallmark symptoms. Toxic transthyretin clumps also can build up in the heart muscle, resulting in damage to the heart, or cardiomyopathy.
Onpattro is designed to reduce production of the abnormal protein through a process called RNA interference, or simply RNAi.
When the TTR gene is read to make the transthyretin protein, the genetic code is copied from the cell’s DNA into a temporary molecule called messenger RNA (mRNA). The mRNA then is shipped to the cell’s protein-making machines, called ribosomes, which use the mRNA as a template to make transthyretin.
Onpattro is a small interfering RNA (siRNA) molecule that targets TTR’s mRNA molecules, thereby interfering with transthyretin production and lowering the levels of these toxic protein clumps. The therapy uses lipid nanoparticles, or tiny bubble-like structures made of fatty molecules, to deliver the siRNA to liver cells, the main producers of transthyretin in the body.
It’s the predecessor of Alnylam’s Amvuttra (vutrisiran), an FAP-approved therapy that works essentially the same but was designed to last longer in the body, thereby allowing for less frequent dosing.
Who can take Onpattro?
Onpattro was approved by the FDA in August 2018 to treat adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis, which is another term for FAP. The decision made Onpattro the first therapy to be approved for FAP and the first approved siRNA-based treatment in the U.S.
In Canada, Onpattro is approved for the same indication. Meanwhile, its approvals in Brazil and Switzerland, as well as in the European Union, cover adults with hereditary transthyretin-mediated amyloidosis with stage 1 or stage 2 polyneuropathy. The treatment also is cleared in Japan for people with FAP, regardless of age.
Who should not take Onpattro?
The prescribing information for Onpattro does not list any contraindications.
How is Onpattro administered?
Onpattro is available in single-dose vials containing 10 mg of the active therapy in 5 mL of an off-white, opalescent solution. It is given by infusion into the bloodstream every three weeks by a healthcare professional.
Prior to infusion, which lasts about 80 minutes, the therapy must be diluted and filtered. The recommended dosage is:
- 0.3 mg/kg for patients weighing less than 100 kg (about 220 lbs.)
- 30 mg for those weighing 100 kg or more.
At least one hour before each Onpattro infusion, patients should receive medications to reduce the risk of infusion-related reactions. Some options are an intravenous corticosteroid, such as dexamethasone, oral acetaminophen — a fever-reducing medication sold as Tylenol, among others — and intravenous antihistamines, including an H1 blocker such as diphenhydramine and an H2 blocker such as ranitidine.
When a scheduled Onpattro dose is missed, an infusion should be given as soon as possible. If the therapy is given within three days of the missed dose, the next infusion can be given according to the original schedule. But if there’s a delay of more than three days, the next dose should be given every three weeks counting from the actual date of the delayed infusion.
Onpattro in clinical trials
Onpattro’s approvals were based mainly on data from a global, placebo-controlled Phase 3 clinical trial called APOLLO (NCT01960348).
APOLLO
The APOLLO study enrolled 225 adults with FAP, ages 18 to 85. Participants were randomly assigned to receive Onpattro, at a dose of 0.3 mg/kg, or a placebo every three weeks for 18 months.
The study’s main goal was to assess the effect of Onpattro on the modified Neuropathy Impairment Score +7 (mNIS+7), which assesses disability associated with neuropathy, or nerve damage. Higher mNIS+7 scores reflect greater impairment.
Trial results showed that, in the placebo group, mNIS+7 scores increased by an average of 28 points over the course of the 18-month trial, which is consistent with how neuropathy tends to worsen over time in untreated FAP patients. By contrast, patients on Onpattro showed a 6-point drop on the mNIS+7 score, on average, indicating less neuropathy. The score difference between the groups was statistically significant, meeting the trial’s main goal.
These changes in the mNIS+7 score were associated with changes in blood transthyretin levels, with patients on Onpattro experiencing a median 81% reduction in protein levels over the trial’s course.
Also, a significantly greater proportion of Onpattro-treated patients experienced a mNIS+7 score reduction relative to those on a placebo (56% vs. 4%). For those who didn’t show an improvement, treatment with Onpattro still resulted in a milder increase in mNIS+7 scores than among those on the placebo (9.9 vs. 26.5 points), indicating slower disease progression.
Onpattro’s superiority was observed across all patient subgroups, regardless of age, sex, ethnicity, and geographical region, as well as any individual disease-causing mutations or FAP stage.
Quality of life, measured by the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QoL-DN), showed similar trends: It improved by an average of 6.7 points in the Onpattro group and worsened by 14.4 points in the placebo group.
Onpattro also significantly outperformed the placebo on other secondary goals, including measures of physical function and nutrition.
Subsequent analyses of APOLLO data also indicated that Onpattro treatment significantly reduced blood levels of neurofilament light chain (NfL), a marker of nerve damage, while levels of this biomarker increased in the placebo group.
Extension study
Participants who completed APOLLO had the option to enroll in an open-label extension study (NCT02510261) in which all were given Onpattro and monitored for long-term outcomes. The extension study also included adults with FAP who had completed previous Onpattro Phase 2 trials.
Polyneuropathy continued to ease with long-term Onpattro treatment, the data indicated. Among patients who had been on Onpattro since APOLLO, mNIS+7 scores decreased by an average of four points after one year in the extension. After two years, the reduction was nearly five points, indicating sustained polyneuropathy lessening.
Among patients who were given a placebo during APOLLO, mNIS+7 scores were on average mostly unchanged in the first two years of the extension study, suggesting a stabilizing of disease immediately after starting on Onpattro. Quality of life measures and biomarkers like NfL also showed sustained improvement in the extension study.
Phase 3b trial
Because APOLLO excluded patients who had previously received a liver transplant, Alnylam sponsored a European Phase 3b study (NCT03862807) in which 23 FAP patients received Onpattro treatment for a year following a liver transplant.
The results showed Onpattro was associated with a significant reduction in blood transthyretin levels — by about 90% — and also with less neuropathy and better quality of life. The therapy’s safety profile in these patients was consistent with that reported in other studies.
Common side effects of Onpattro
The most common side effects of Onpattro include:
- upper respiratory tract infections, including colds, sinus infections, and nasal congestion
- infusion-related reactions.
Infusion-related reactions
Some patients can experience an infusion-related reaction during or soon after Onpattro is administered. The symptoms of such reactions may include flushing, back pain, nausea, headache, severe low blood pressure, and fainting.
To reduce the risk of infusion reactions, patients should be premedicated with a corticosteroid, acetaminophen, and/or antihistamines before each Onpattro dose.
Patients also should be monitored for signs and symptoms of infusion-related reactions during the infusion. If such a reaction occurs, the infusion may be slowed or paused, and appropriate supportive care may be given. If paused, the infusion may be resumed at a slower rate once symptoms resolve, unless the reaction was serious or life-threatening, in which case treatment should not be resumed.
For some patients experiencing infusion reactions, a slower infusion rate or higher doses of the premedications in subsequent infusions may help reduce the risk of reactions.
Vitamin A supplementation
Because Onpattro works by reducing transthyretin, a vitamin A transporter, the therapy can lower blood vitamin A levels. It’s recommended that patients on Onpattro take vitamin A supplements at the recommended daily dose, which is 900 micrograms for adult men and 700 micrograms for adult women. The dosage of vitamin A supplements should not exceed the recommended daily allowance, regardless of measured blood vitamin A levels, as these do not reflect total levels in the body.
Patients who experience eye symptoms of vitamin A deficiency, such as difficulty seeing at night, should talk with their physicians and be referred to an ophthalmologist.
Use in pregnancy and breastfeeding
There are no data available on the use of Onpattro in people who are pregnant. However, as Onpattro may cause reductions in vitamin A, which is essential for normal fetal development, the therapy may potentially cause damage to the fetus.
Preclinical studies assessing the effects of the therapy when administered to pregnant animals have reported no notable complications for the developing fetuses in rats, but problems for both the fetus and mother have been observed in rabbits.
It also remains unclear whether Onpattro can be found in human breast milk, or if it has effects on nursing infants or on milk production. Data from rat studies suggest that its active agent, patisiran, is not secreted in milk, though some of the fatty molecules used in the therapy are. The health consequences of these molecules in milk are unknown.
Use of Onpattro in pregnancy and breastfeeding should be made on a case-by-case basis, evaluating the potential benefits and risks of treatment. Alnylam is now running a pregnancy surveillance program (NCT05040373) to collect data on Onpattro’s use during pregnancy and its effects on maternal and fetal outcomes.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Liver transplant, therapies mean longer life with hATTR, data show
- Onpattro stabilizes FAP progression in 2 common genetic variants
- New study tests nucresiran’s potential to slow nerve damage
- Our role as patient advocates is vital, but funding remains an issue
- Gene therapy study placed on hold after elderly man passes away
Related articles