Diflunisal for FAP
Last updated March 28, 2024, by Marisa Wexler, MS

What is diflunisal for FAP?
Diflunisal is an oral medication that’s been investigated as a potential treatment for familial amyloid polyneuropathy (FAP) and is sometimes used off-label to slow disease progression in this patient population.
The medication is a nonsteroidal anti-inflammatory drug that was approved in the U.S. in 1982 and is indicated for mild to moderate pain and some forms of arthritis.
It was originally developed by Merck and sold under the brand name Dolobid, but that brand was discontinued in the U.S. for reasons other than safety or effectiveness. Only generic versions of the therapy are currently available.
Therapy snapshot
| Treatment name: | Diflunisal |
| Administration: | Being tested in FAP as an oral tablet formulation |
| Clinical testing: | Completed a Phase 2/3 clinical trial |
How does diflunisal work in FAP?
FAP is caused by mutations in the TTR gene, which provides instructions for making the protein transthyretin (TTR). The protein normally exists as a tetramer, or a complex of four TTR proteins bound together, but in FAP, a mutated protein is produced that isn’t stable in the normal tetramer form.
The unstable protein tends instead to form abnormal clumps called amyloid fibrils that accumulate to toxic levels in tissues, mostly in nerves, leading to FAP symptoms.
Besides its anti-inflammatory effects, diflunisal has been shown to stabilize the tetramer of both healthy and mutated TTR proteins. As such, the therapy should prevent the mutated TTR protein from forming FAP-driving toxic clumps, slowing disease progression.
How is diflunisal administered in FAP?
Diflunisal is available as film-coated tablets, each containing 500 mg of the active ingredient.
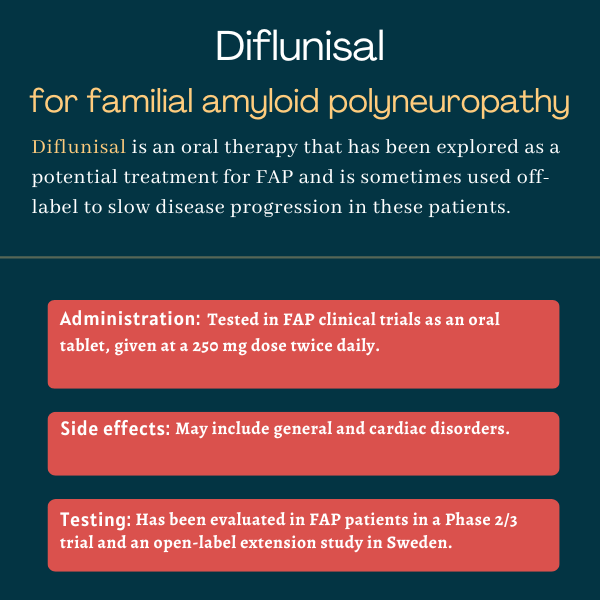
In FAP clinical trials, the medication has been administered as oral tablets, at 250 mg twice daily. Due to promising results with that dosing regimen, the medication is currently prescribed off-label in several countries, at the same dose.
Diflunisal in FAP clinical trials
Diflunisal’s safety and effectiveness were evaluated in FAP patients in a single Phase 2/3 trial (NCT00294671) sponsored by Boston University.
Phase 2/3 trial
The study ran from 2006 to 2012, and enrolled 130 adults, ages 18-75, at eight sites in the U.S., England, Sweden, Italy, and Japan. Participants were randomly assigned to receive 250 mg diflunisal or a placebo twice daily for two years.
The study was designed to test whether diflunisal could slow the progression of nerve damage, or neuropathy, in FAP patients. It’s main goal was to assess changes on the Neurologic Impairment Score +7 (NIS+7), a measure of neuropathy severity that ranges from 0 to 270 points, with higher values reflecting worse neuropathy. In untreated FAP patients, NIS+7 scores tend to increase over time as the disease progresses.
Results showed that average NIS+7 scores increased by 8.2 points in diflunisal-treated patients after two years compared with a 26.3-point increase in patients on a placebo. This reflected a statistically significant group difference of 18 points, indicating disease progression was slower for those given diflunisal.
A significantly greater proportion of patients on diflunisal also showed stable disease at two years, defined as a NIS+7 score increase of less than 2 points, relative to those on a placebo (29.7% vs. 9.4%). This reflected about a three times higher risk of experiencing disease stabilization with diflunisal.
Scores on a generalized measure of life quality called the 36-Item Short-Form Health Survey (SF-36) also favored diflunisal over a placebo. After two years, average scores of both physical and mental SF-36 domains worsened in the placebo group, but improved in patients treated with diflunisal, suggesting the therapy helped improve patients’ quality of life.
A total of 67 patients discontinued the trial before completing the full two years of treatment, mostly due to disease progression or liver transplant, but analyses revealed the dropout rate due to disease progression was significantly higher in the placebo group, again supporting the superiority of diflunisal.
Open-label extension study
After completing the Phase 2/3 trial, participants at the Swedish site could enroll in an open-label extension study, called DFNS01 (NCT01432587), wherein all received diflunisal for up to two years. The study also included people with disease-causing TTR mutations who hadn’t received previous treatment.
A total of 54 patients were enrolled, including 14 who had received diflunisal for up to two years in the previous study. However, only 17 participants (31%) completed two years of follow-up, most often due to study closure.
Although the high dropout rates were a notable limitation, available data were generally consistent with findings from the initial Phase 2/3 trial. Specifically, total scores on the Kumamoto Neurologic Scale, which measures several aspects of FAP disease, remained stable for two years. This was also true for domains of sensory neuropathy, neuropathy-related problems in involuntary bodily processes, and organ dysfunction.
Scores of the motor neuropathy domain significantly worsened over the study, however, indicating that, while diflunisal may slow FAP’s progression, it doesn’t stop it entirely.
The seven patients who had received diflunisal in the previous trial and completed DFNS01’s two-year treatment period showed no significant changes over time for any of the outcome measures.
Common side effects of diflunisal
In the Phase 2/3 trial that tested diflunisal against a placebo in FAP patients, rates of drug-related side effects were generally similar between the two groups, although general and cardiac disorders were more frequently reported in the diflunisal group.
The chronic use of nonsteroidal anti-inflammatory drugs such as diflunisal is associated with a higher risk of side effects affecting the gastrointestinal tract, kidneys, or heart, however.
Therefore, diflunisal may be not recommended for FAP patients with heart involvement and regular monitoring for these potential side effects may be advised when the therapy is used off-label. Patients must discuss the potential risks with their healthcare team before taking diflunisal.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Related articles