Amvuttra (vutrisiran) for FAP
Last updated April 15, 2025, by Lindsey Shapiro, PhD

What is Amvuttra for FAP?
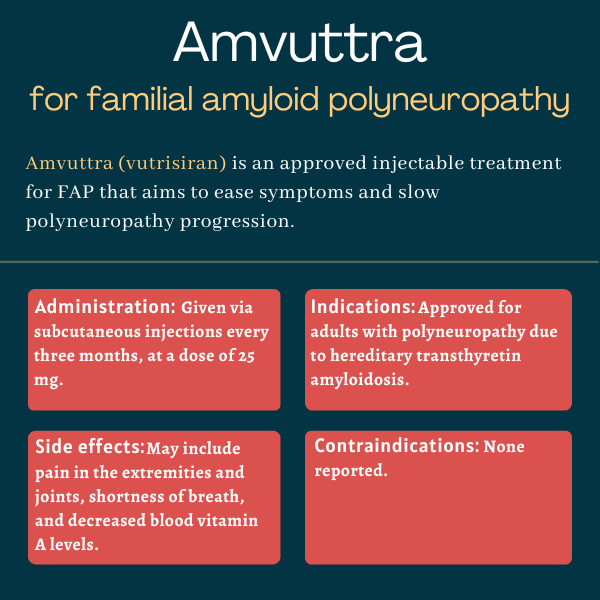
Amvuttra (vutrisiran) is an injectable treatment approved for adults with familial amyloid polyneuropathy (FAP), also known as hereditary transthyretin amyloidosis with polyneuropathy.
The therapy, which is given via a subcutaneous (under-the-skin) injection, works to ease symptoms of polyneuropathy — those resulting from the damage to nerves outside the brain and spinal cord — and slow or prevent disease progression.
Previously known as ALN-TTRsc02, it was developed and is marketed by Alnylam Pharmaceuticals. Amvuttra is also approved in the U.S. for adults with transthyretin-mediated amyloidosis with cardiomyopathy (ATTR-CM), a FAP-related condition marked by heart damage.
Therapy snapshot
| Brand name: | Amvuttra |
| Chemical name: | Vutrisiran |
| Usage: | Used to ease symptoms and slow or halt disease progression in adults with FAP |
| Administration: | Subcutaneous injection |
How does Amvuttra work?
FAP is caused by mutations in the TTR gene that provides instructions for making the protein transthyretin. As a result of these mutations, an abnormal form of transthyretin is produced, which tends to form toxic clumps, called amyloid fibrils, that build up in the body’s tissues, particularly in the nerves outside the brain and spinal cord. In some cases, these toxic aggregates accumulate in heart tissue.
To produce the transthyretin protein, TTR’s genetic code is used to form an intermediate molecule called messenger RNA (mRNA). The mRNA is then shipped from the nucleus to the cell’s protein-making machinery, where it is used as a template for making transthyretin.
Amvuttra is second-generation small interfering RNA (siRNA) that’s designed to bind to TTR’s mRNA molecules and promote their destruction through a natural process called RNA interference. The therapy was modified to be attached to three single sugar molecules called GalNAc that improve its delivery to liver cells, the body’s main producers of transthyretin.
By reducing the levels of TTR’s mRNA molecules in liver cells, the production of the faulty transthyretin protein that causes FAP is reduced, preventing further nerve damage, or neuropathy.
The therapy’s mechanism of action is similar to that of Onpattro (patisiran), an older FAP approved treatment developed by Alnylam that is given every three weeks directly into the bloodstream. Amvuttra’s chemical modifications, however, allow for subcutaneous administration and greater stability in the body, meaning less frequent dosing relative to its predecessor.
Who can take Amvuttra?
Amvuttra was approved by the U.S. Food and Drug Administration in June 2022 for treating adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis, another name for FAP.
A few months later, the therapy also was cleared for use in the European Union for adult FAP patients with stage 1 or stage 2 polyneuropathy. Amvuttra is approved for that same indication in Canada.
Who should not take Amvuttra?
There are no known contraindications for Amvuttra’s use.
How is Amvuttra administered?
Amvuttra is available in single-dose, pre-filled syringes containing 25 mg of the therapy in 0.5 mL of a colorless-to-yellow solution. The recommended dose is 25 mg, given once every three months as a subcutaneous injection by a healthcare professional.
If a dose is missed, Amvuttra should be administered as soon as possible, and dosing every three months should be resumed from the most recent injection.
A twice-yearly administration of Amvuttra at 50 mg is being evaluated in clinical trials.
Amvuttra in clinical trials
Regulatory approvals of Amvuttra were based mainly on results from a global Phase 3 clinical trial called HELIOS-A (NCT03759379).
HELIOS-A
The Alnylam-sponsored HELIOS-A study evaluated the safety and efficacy of Amvuttra in 164 adults with FAP at more than 50 sites globally.
Participants were randomly assigned to receive either Amvuttra at the now approved regimen — a subcutaneous injection of 25 mg once every three months — or Onpattro, given as an into-the-vein infusion once every three weeks. In both groups, treatment was given for up to 18 months (1.5 years).
The results were compared with those from a group of FAP patients who had received a placebo in the previous Phase 3 APOLLO trial (NCT01960348), which helped support Onpattro’s approval.
The trial’s main goal was to assess changes in neuropathy symptoms after nine months of treatment, measured using the modified Neurologic Impairment Score +7 (mNIS+7).
Amvuttra-treated patients saw a 2.24-point decline in mNIS+7 scores after nine months, reflecting an easing in neuropathy symptoms. By contrast, FAP patients in the external placebo group saw a nearly 15-point increase in mNIS+7 scores, indicating worsening neuropathy, over the same period. The score change difference between these two groups was statistically significant, meaning the main goal was met.
Amvuttra’s superiority was maintained after 1.5 years of treatment, with patients on the experimental therapy achieving a 0.46-point decrease in mNIS+7 scores, compared with a 28.09-point increase in the external placebo group. Also, a significantly greater proportion of patients on Amvuttra had mNIS+7 improvements than those on a placebo (48.3% vs. 3.9%).
Treatment with Amvuttra also led to improvements in quality of life, walking speed, and nutritional status, as well as reductions in overall disability status. Sustained decreases in blood transthyretin levels also were seen starting at three weeks and for up to 18 months, with levels dropping by more than 80%.
Subsequent analyses of HELIOS-A data indicated that blood levels of neurofilament light chain (NfL), a marker of nerve damage, were significantly reduced with both Amvuttra and Onpattro. Moreover, Amvuttra-associated transthyretin reductions and clinical improvements were generally comparable to those achieved with Onpattro over about 1.5 years of treatment.
Exploratory 18-month data also suggested Amvuttra could lessen heart damage in patients with heart involvement, as reflected by a reduction in NT-proBNP, a biomarker of heart strain, and improvement in other heart parameters.
Extension study
Participants completing HELIOS-A’s 18-month treatment period were able to enter the trial’s ongoing randomized treatment extension phase, where all are receiving Amvuttra for an additional 18 months. In the extension phase, patients were randomly assigned to receive either the approved regimen or a new twice-yearly regimen, with a 50 mg injection given every six months. The study is expected to end in late 2026.
Common side effects of Amvuttra
The most common side effects reported with Amvuttra in clinical trials include:
- pain in the arms or legs
- joint pain
- shortness of breath
- decreased levels of vitamin A in the blood.
Decreased vitamin A levels
Due to transthyretin’s role in transporting vitamin A throughout the body, Amvuttra leads to a decrease in blood levels of vitamin A. Daily vitamin A supplements at the recommended daily dose, but no higher, are advised for all patients taking Amvuttra. If patients have eye-related symptoms, such as night blindness, that suggest a vitamin A deficiency, they should be referred to an ophthalmologist.
Use in pregnancy and breastfeeding
Pregnant and breastfeeding women were not included in clinical trials of Amvuttra, so there’s no information about whether the therapy is safe during pregnancy or breastfeeding. Further, vitamin A is essential for normal embryonic development, but the effects of low blood levels of vitamin A and the use of vitamin A supplementation on a developing fetus are unknown.
Some animal studies have indicated Amvuttra may have harmful effects on a developing fetus at doses also associated with maternal toxicity, while others have seen no adverse developmental effects on the fetus.
Patients who are pregnant or nursing, or wish to become pregnant or breastfeed, while using Amvuttra should discuss this issue with their physician.
FAP News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Treatment switches in FAP are common, reasons numerous: Study
- Phase 3 trial is tracking use of acoramidis to block ATTR onset
- I wish my country could benefit from gains in amyloidosis treatment
- Long-term Amvuttra treatment safe, pooled trial analysis shows
- Gene therapy nex-z lowers FAP protein levels for 3 years
Related articles






